Pharmaceutical Analysis-III
Multiple Choice Questions
5 Pages
MPS
Contributed by
Mahmood Prasoon Sarna
Loading
 Subject: Pharmaceutical Analysis-III Class: L. Y. B. PharmSemester: VII Syllabus: CBSCSMultiple Choice Questions1. In which chromatography stationary phase is more polar than mobile phase?A. Ion exchange chromatographyB. Normal phase chromatographyC. Reversed chromatographyD. Size exclusion chromatography2. In which type of chromatography, the stationary phase is held in a narrow tube andthe mobile phase is forced through it under pressure?A. Column chromatographyB. Planar chromatographyC. Liquid chromatographyD. Gas chromatography3. Which of the following guidelines are applicable to Analytical Method validationA. ICH Q1B. ICH Q2C. ICH Q3D. ICH Q44. In thin layer chromatography, the stationary phase is made of _________ and themobile phase is made of _________A. Solid, liquidB. Liquid, liquidC. Liquid, gasD. Solid, gas5. Which of the following multicomponent analysis technique involves estimation ofcomponents that show change in spectra in different pH mediumA. Simultaneous equationB. Derivative spectroscopyC. Q Absorbance ratioD. Difference spectroscopy
Subject: Pharmaceutical Analysis-III Class: L. Y. B. PharmSemester: VII Syllabus: CBSCSMultiple Choice Questions1. In which chromatography stationary phase is more polar than mobile phase?A. Ion exchange chromatographyB. Normal phase chromatographyC. Reversed chromatographyD. Size exclusion chromatography2. In which type of chromatography, the stationary phase is held in a narrow tube andthe mobile phase is forced through it under pressure?A. Column chromatographyB. Planar chromatographyC. Liquid chromatographyD. Gas chromatography3. Which of the following guidelines are applicable to Analytical Method validationA. ICH Q1B. ICH Q2C. ICH Q3D. ICH Q44. In thin layer chromatography, the stationary phase is made of _________ and themobile phase is made of _________A. Solid, liquidB. Liquid, liquidC. Liquid, gasD. Solid, gas5. Which of the following multicomponent analysis technique involves estimation ofcomponents that show change in spectra in different pH mediumA. Simultaneous equationB. Derivative spectroscopyC. Q Absorbance ratioD. Difference spectroscopyPage 1
 Subject: Pharmaceutical Analysis-III Class: L. Y. B. PharmSemester: VII Syllabus: CBSCS6. In which of the following type of paper chromatography does the mobile phase movehorizontally over a circular sheet of paper?A. Ascending paper chromatographyB. Descending paper chromatographyC. Radial paper chromatographyD. Ascending – descending chromatography7. In size exclusion chromatography, solute molecules are separated based on _________A. Molecular geometry and sizeB. Molecular compositionC. Molecular phaseD. Molecular formula8. Ion exchange chromatography is based on?A. Electrostatic attractionB. Electrical mobility of ionic speciesC. Partition chromatographyD. Adsorption chromatography9. Which of the following is an example of bulk property or general detector in HPLCA. Fluorescence detectorB. Refractive index detectorC. Electrochemical detectorD. UV-Visible detector10. Which of the following is used as a carrier gas in gas chromatographyA. Carbon dioxideB. OxygenC. HeliumD. Methane
Subject: Pharmaceutical Analysis-III Class: L. Y. B. PharmSemester: VII Syllabus: CBSCS6. In which of the following type of paper chromatography does the mobile phase movehorizontally over a circular sheet of paper?A. Ascending paper chromatographyB. Descending paper chromatographyC. Radial paper chromatographyD. Ascending – descending chromatography7. In size exclusion chromatography, solute molecules are separated based on _________A. Molecular geometry and sizeB. Molecular compositionC. Molecular phaseD. Molecular formula8. Ion exchange chromatography is based on?A. Electrostatic attractionB. Electrical mobility of ionic speciesC. Partition chromatographyD. Adsorption chromatography9. Which of the following is an example of bulk property or general detector in HPLCA. Fluorescence detectorB. Refractive index detectorC. Electrochemical detectorD. UV-Visible detector10. Which of the following is used as a carrier gas in gas chromatographyA. Carbon dioxideB. OxygenC. HeliumD. MethanePage 2
 Subject: Pharmaceutical Analysis-III Class: L. Y. B. PharmSemester: VII Syllabus: CBSCS11.1H nuclei located near electronegative atoms tend to be ______ relative to1H nucleiA. ShieldedB. DeshieldedC. ResonancedD. Split12. Signal splitting in NMR arises from?A. Shielding effectB. Spin-spin decouplingC. Spin-spin couplingD. Deshielding effecr13. The base peak in mass spectrum is?A. The lowest mass peakB. The peak corresponding to the parent ionC. The highest mass peakD. The peak set to 100% relative intensity14. Which one of the following is necessary for mass spectrometry to occur?A. Loss of an electronB. Change of alignment of a proton in a magnetic fieldC. A molecular vibrationD. Excitation of an electron from the ground state to a higher energy state15. Column efficiency is measured in terms of number of theoretical plates, which is:A. Inversely related to square root of height equivalent to theoretical platesB. Directly related to square root of height equivalent to theoretical platesC. Directly related to height equivalent to theoretical platesD. Inversely related to height equivalent to theoretical plates
Subject: Pharmaceutical Analysis-III Class: L. Y. B. PharmSemester: VII Syllabus: CBSCS11.1H nuclei located near electronegative atoms tend to be ______ relative to1H nucleiA. ShieldedB. DeshieldedC. ResonancedD. Split12. Signal splitting in NMR arises from?A. Shielding effectB. Spin-spin decouplingC. Spin-spin couplingD. Deshielding effecr13. The base peak in mass spectrum is?A. The lowest mass peakB. The peak corresponding to the parent ionC. The highest mass peakD. The peak set to 100% relative intensity14. Which one of the following is necessary for mass spectrometry to occur?A. Loss of an electronB. Change of alignment of a proton in a magnetic fieldC. A molecular vibrationD. Excitation of an electron from the ground state to a higher energy state15. Column efficiency is measured in terms of number of theoretical plates, which is:A. Inversely related to square root of height equivalent to theoretical platesB. Directly related to square root of height equivalent to theoretical platesC. Directly related to height equivalent to theoretical platesD. Inversely related to height equivalent to theoretical platesPage 3
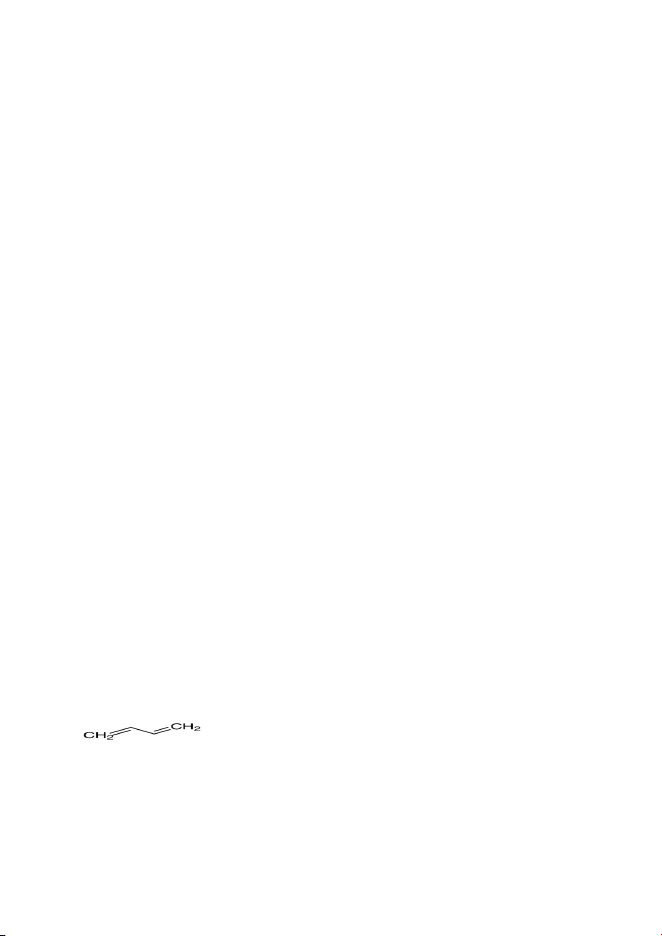 Subject: Pharmaceutical Analysis-III Class: L. Y. B. PharmSemester: VII Syllabus: CBSCS16. In reverse phase HPLC, there is aA. Non-polar solvent/polar columnB. Polar solvent/Non-polar columnC. Polar solvent/Polar columnD. Non-polar solvent/Non-polar column17. In a chromatographic separation, which of the following is most appropriate for thequalitative analysis of a substance?A. Taking factorB. Capacity factorC. Retention timeD. Resolution18. The basis of the technique of chromatography for separating components of amixture is?A. the differing movement of particles of different mass in an electrical fieldB. the interaction of the components with a stationary and a mobile phasesC. the absorption of infrared radiation by the components.D. the deflection of charged particles in a magnetic field.19. HPLC is an abbreviation for?A. High Profit Liquid ChromatographyB. High Pressure Liquid ChromatographyC. Higher Performance Low ChromatographyD. Higher Profit Low Chromatography20. The base value for the following compound isA. 214B. 253C. 217D. 202
Subject: Pharmaceutical Analysis-III Class: L. Y. B. PharmSemester: VII Syllabus: CBSCS16. In reverse phase HPLC, there is aA. Non-polar solvent/polar columnB. Polar solvent/Non-polar columnC. Polar solvent/Polar columnD. Non-polar solvent/Non-polar column17. In a chromatographic separation, which of the following is most appropriate for thequalitative analysis of a substance?A. Taking factorB. Capacity factorC. Retention timeD. Resolution18. The basis of the technique of chromatography for separating components of amixture is?A. the differing movement of particles of different mass in an electrical fieldB. the interaction of the components with a stationary and a mobile phasesC. the absorption of infrared radiation by the components.D. the deflection of charged particles in a magnetic field.19. HPLC is an abbreviation for?A. High Profit Liquid ChromatographyB. High Pressure Liquid ChromatographyC. Higher Performance Low ChromatographyD. Higher Profit Low Chromatography20. The base value for the following compound isA. 214B. 253C. 217D. 202Page 4
 Subject: Pharmaceutical Analysis-III Class: L. Y. B. PharmSemester: VII Syllabus: CBSCS21. In IR spectroscopy, the wavenumber for nitrile group is observed in the range ofA. 3500-3300 cm-1B. 2200-2100 cm-1C. 1740-1650 cm-1D. 3000-2800 cm-122. Which of the following techniques would be most useful to identify as well asquantify the presence of a known impurity in a drug substance?A. NMRB. MSC. IRD. HPLC23. The results for precision studies in Analytical Method Validation, are expressed interms of?A. % Relative errorB. Correlation coefficientC. % Relative standard deviationD. Mean24. Which of the following is used as a spraying reagent in paper chromatography?A. conc. HClB. NaCl solutionC. Ninhydrin solutionD. CuSO4 solution25. In mass spectrometry, fragmentation of ions is achieved through?A. IonizationB. SplittingC. SolubilizationD. Coupling
Subject: Pharmaceutical Analysis-III Class: L. Y. B. PharmSemester: VII Syllabus: CBSCS21. In IR spectroscopy, the wavenumber for nitrile group is observed in the range ofA. 3500-3300 cm-1B. 2200-2100 cm-1C. 1740-1650 cm-1D. 3000-2800 cm-122. Which of the following techniques would be most useful to identify as well asquantify the presence of a known impurity in a drug substance?A. NMRB. MSC. IRD. HPLC23. The results for precision studies in Analytical Method Validation, are expressed interms of?A. % Relative errorB. Correlation coefficientC. % Relative standard deviationD. Mean24. Which of the following is used as a spraying reagent in paper chromatography?A. conc. HClB. NaCl solutionC. Ninhydrin solutionD. CuSO4 solution25. In mass spectrometry, fragmentation of ions is achieved through?A. IonizationB. SplittingC. SolubilizationD. CouplingPage 5
Related documents:
- Zoology model question paper 2 - Question Paper
- GE6075 Professional Ethics in Engineering - Notes
- HISTORY II 2016 question paper - Question Paper
- Social Construction Of Gender Unit-5 Quetions with answers - Question Bank
- Bcs 304 Data Communication And Networking MCQs - MCQ
- FULL TEST – I Paper 2 - Question Paper
- Statistics (Paper I) 2017 Question Paper - Question Paper
- GENERAL STUDIES Test Booklet - Question Paper
- Unconventional Machining Processes Solved MCQs - MCQ
- Data Mining MCQs - MCQ
- Basics Economics Studies MCQs - MCQ
- Logic (Mathematical Reasoning) (Solved MCQs and Notes) - Notes
- Questions - MCQ
- Advanced Corporate Accounting - Notes
- HISTORY II 2020 question paper - Question Paper
- Labor Law MCQs with Answers - MCQ
- NIRBHAYA FUND - Notes
- Data Structure Solved MCQs - MCQ
- Banking regulation and operations (BRO) - Notes
- Sociology (Paper I) 2019 Question Paper - Question Paper
